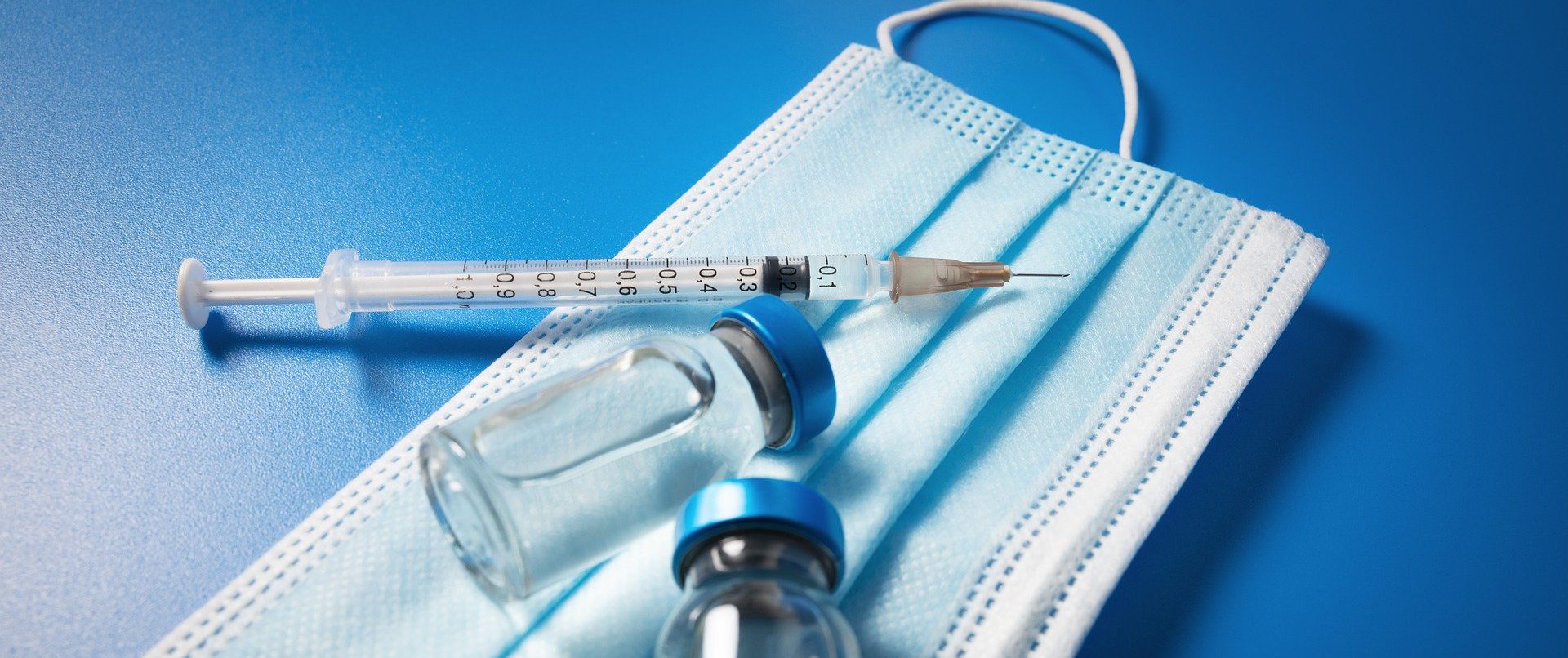
Best practices as recommended by WHO and United States Pharmacopoeia are being implemented for performing tests on therapeutic goods.
Recent Updates
- Training on Software for Electronic Submission of Applications for Quota Allocations and Functions of Controlled Drugs DivisionNovember 14, 2025
- Draft Guideline for Lot Release of Human Vaccines, Blood Products (Plasma Derivatives Medicinal Products) and Anti-Sera (Edition 03)November 13, 2025
- Decisions of the 105th Meeting of the Medical Device Board (MDB) held on 23-10-2025 (Manual Applications)November 13, 2025
- 108. Recall Alert (Veterinary Drug Product) – Melovetz 10 Injection (Batch # 2599063)November 12, 2025
- 107. Recall Alert – (Class-I) Injection Isobaj (Batch # IB-0925)November 12, 2025
- 106.Recall Alert – (Class-III) Tablet Cekamol 500 mg (Batch # T-5014)November 12, 2025
- Minutes of the 349th Meeting of the Registration BoardNovember 10, 2025
- Implementation of Pre-Import Clearance Mechanism under DRAP-PSW Gateway System for Import of Drugs (Raw Materials and Finished Products)November 6, 2025
- Decisions of the 104th Meeting of the Medical Device Board (MDB) held on 16-10-2025 (Part-II: Manual Applications)November 4, 2025
- 105. Recall Alert – Drug Products for Human Use Declared SubstandardOctober 30, 2025
- Show all updates
Chief Executive Officer
 Dr. Obaidullah
Dr. Obaidullah

E-Services
With the effective use of Information Technology, we have implemented a number of online services and continually working for more. Click here to browse available services.

Guidelines
Regulatory compliance is essential and indispensable in order to assure the safety, quality and efficacy of therapeutic goods. Guidance documents published by the Authority are meant to explain the current interpretation or policy for better understanding of the industry and

Quality Control Labs
For ensuring quality therapeutic goods, a network of quality control laboratories has been established. DRAP approved products and suspect therapeutic goods are tested for their quality in accordance with best international practices. To keep the citizens safe from substandard and

Therapeutic Products Recalls
The Recalled Product Index provides access to consumers, healthcare professionals, industry, hospitals and sale outlets related to the information of recalled action taken for defective therapeutic goods. The following section provides an index of recalled actions in which a public

Health Professional Alerts
DRAP’s issues safety updates and alerts related to the therapeutic goods in order to ensure that healthcare professionals and other stakeholders have timely access to the relevant information on safety, quality and efficacy issues or benefit/risk evaluations of the drugs,

Medicine Availability
The Drug Regulatory Authority of Pakistan (DRAP) is committed to safeguard public health by ensuring the consistent availability of safe, efficacious, and quality medicines nationwide. Recognizing the critical impact of drug shortages, particularly of essential and lifesaving medicines, on patient
Lot Release Application System
We've launched Lot Release Application System. Please use following link to submit your lot release applications for biological products.
New system for the applications of Medical Devices
We are thrilled to announce that the new MDMC Licensing and Registration module is officially launched. This enhanced module has been designed to offer a more streamlined, efficient, and user-friendly experience for managing all MDMC licensing and registration activities.
Do you know?
DRAP ensures that every drug, medical device or cosmetic, alternative medicine and health product must have a certain standard of quality and is safe and effective for your use. See in links below how it is done and what to do in case you find a suspect therapeutic good.